Spin Q ms is 12 or -12 describing the spin of an electron more for bookkeeping than physical meaning magnetic Q ml represents the spacial direction of the orbital ie. A It is a spherical or dumbbell-shaped route traced by the electron in its rapid movement.

Orbital Chemistry And Physics Britannica
In atomic theory and quantum mechanics an atomic orbital is a mathematical function describing the location and wave-like behavior of an electron in an atom.
. What is an orbital best described as. A single space within an atom that contains all. B A three-dimensional mathematical description of the most likely location of an electron in an atom.
Which statement best describes an occupied orbital according to the quantum mechanical model. Science Chemistry QA Library Which statement best describes an occupied orbital according to the quantum mechanical model. Electron configuration of iodine Kr5s24d105p5.
Describe the shape of a d orbital. An orbital is the area around an atom where the electron or electrons are most likely to be. Which term best describes an orbital.
Hydrogen has one electron in a 1s orbital and we write its electron configuration as 1s 1. They are effectively a map of the electrons for a given atom. A A space in an atom where an electron is most likely to be found b Space where electrons are unlikely to be found in an atom.
Principle quant n determines size of orbital. Which of the following best describes an orbital. Which of the following statements best describes an atomic orbital.
A exact B probability C Bohr model D path. C The two-dimensional circular orbits electrons take around the nucleus. A a space in an atom where an electron is most likely to be found b space where electrons are unlikely to be found in an atom.
We do this with something called electron configurations. The space in an atom where an electron is most likely to be found d. Angular moment Q l represents the shape.
The electron with quantum numbers n 3 l 2 me 2 m -12 is the last electron to be. Which of the following best describes an orbital. C It is a region in space in which the.
B It is a region in space that has a precise shape and is completely filled hva dense electron cloud. We look at the four quantum numbers for a given electron and then assign that electron to a specific orbital. The s-orbital is 0 the p-orbital is 1 d-orbital 2 f-orbital 3.
Which of the following best describes an orbital. They are boundless space and have definite energy. A space where electrons are unlikely to be found in an atom B space which may contain electrons protons andor neutrons C the space in an atom where an electron is most likely to be found D small walled spheres that contain electrons E a single space within an atom that contains all.
D No two electrons can have. Use the periodic table to write electron configurations for each element. An atomic orbital is a mathematical term in atomic theory and quantum mechanics that describes the position and wavelike behaviour of an electron in an atom.
Space where electrons are unlikely to be found in an atom b. In lithium the electron configuration is 1s2 2s 1 which tells us that during ionization an electron is being removed from a 2s orbital. Atomic orbital is the physical region or a three dimensional space where the probability of finding the electron is more than 90 which also means that the space where an electron is most likely to be found.
This orbital is equivalent to the innermost electron shell of the Bohr model of the atom. The number of orientations in 3-dimensional space an orbital can have is defined by the magnetic quantum number ml. Option A best describes atomic orbital.
This orbital is spherical in. Al Be In Zr. 4529x104-10 m P Flag question O b.
A a space in an atom where an electron is most likely to be found b space where electrons are unlikely to be found in an atom c space which may contain electrons protons andor neutrons d small walled spheres that contain electrons e a single space within an atom that contains all. D orbital has two. Quantum mechanical calculations tell us that in 2s.
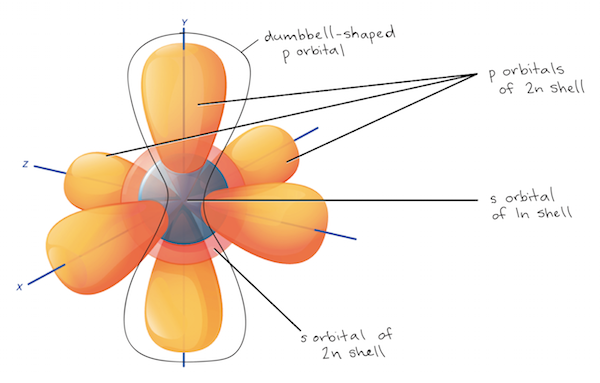
Which of the following best describes an orbital. P subshells are made up of three dumbbell-shaped orbitals. B It is a region in space that has a precise shape and is completely filled by a dense electron cloud.
It is called the 1s orbital because it is spherical around the nucleus. Question 8 Not yet Which comment below best describes the statement. Every such orbital will occupy a maximum of two electrons each having its own quantity of spin.
Three dimensonial standing matter wave that describes the state of an electron in am atom How can you describe the shape of a p-orbital. Write an electron configuration for iodine based on its position in the periodic table. The 1s orbital is always filled before any other orbital.
Question 7 Which best describes the radius of an electron in the n2 orbital of Hydrogen. For any value of n a value of l 0 places that electron in an s orbital. Space which may contain electrons protons andor neutrons c.
An atomic orbital is a mathematical term in atomic theory and quantum mechanics that describes the wave-like behaviour of either one electron or a pair of electrons in an atom. Not yet answered Marked out of 100 - O a. Orientation therefore the answer is.
A It is a spherical or dumbbell-shaped route traced by the electron in its rapid movement. The closest orbital to the nucleus called the 1s orbital can hold up to two electrons. D A mathematical function that describes the movement of atoms in.
A maximum of two electrons each with its own spin quantum number s will occupy each of those orbitals. Helpful 0 Not. When filling orbitals of equal energy two electrons will not occupy the same orbital before each orbital has one unpaired electron.
An electron orbital describes a three-dimensional space where an electron can be found 90 of the time. A A physical barrier around an atom that contains the electron. Small walled spheres that contain electrons e.
Which of the following best describes an orbital. Groups 1 and 2. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atoms nucleus.
Helium has both of its electrons in the 1s orbital 1s 2.

Electronic Orbitals Physical Chemistry Electron Configuration Teaching Chemistry

The Periodic Table Electron Shells And Orbitals Article Khan Academy

Electron Subatomic Particle Chemistry Classroom Teaching Chemistry Chemistry Education

0 Comments